Introduction to Actin
Actin, a vital protein in eukaryotic cells, serves as a elementary part of the cytoskeleton, which supplies structural help and form to cells. It performs a significant function in quite a few mobile processes together with motility, division, and intracellular transport. The actin protein, with a molecular weight of roughly 43 kDa, is very conserved throughout numerous species, emphasizing its evolutionary significance. There are three major isotypes of actin: α-actin, β-actin, and γ-actin. Though these isotypes share over 90% amino acid homology between them and greater than 98% inside a selected isotype group, variations primarily happen within the amino-terminal 30 residues. This area of the protein is located on the periphery of the actin double-helix in F-actin and is essential for interactions with different proteins, together with myosin.
Isotype Variations and Mobile Habits
Actin isotypes exhibit distinct behaviors each in vitro and in vivo, reflecting their specialised capabilities inside completely different mobile contexts. As an example, γ-actin has been noticed to localize in another way in comparison with different isotypes, indicating that the assorted isotypes of actin carry out distinctive roles inside the cell. This specificity is underscored by the differential binding of actin-associated proteins to every isotype. An illustrative instance is the protein ezrin, which reveals a specific affinity for β-actin, highlighting the significance of utilizing the suitable actin isotype in experimental setups to precisely replicate physiological circumstances.
Latest research have demonstrated that isotype-specific interactions of actin-binding proteins (ABPs) can considerably affect mobile processes. As an example, the differential subcellular localization of γ-actin means that particular isotypes are concerned in distinct mobile capabilities, which may affect the interpretation of experimental outcomes if the inaccurate actin isotype is used.
Polymerization of Actin: From G-Actin to F-Actin
Actin exists in two fundamental varieties: globular actin (G-actin) and filamentous actin (F-actin). G-actin is the monomeric, soluble type of the protein, whereas F-actin refers back to the polymerized, filamentous construction.
Polymerization Course of: G-actin polymerizes to kind F-actin underneath physiological circumstances, pushed by the hydrolysis of ATP. F-actin is characterised by a double-helical construction, which imparts intrinsic polarity to the filament. The plus-end (or barbed-end) of the filament displays a better charge of polymerization in comparison with the minus-end (or pointed-end), creating an inherent asymmetry within the filament construction.
Essential Focus (CC): The method of actin polymerization is regulated by the focus of actin monomers. The Essential Focus (CC) is outlined because the monomer focus at which the charges of polymerization and depolymerization are balanced. At concentrations above the CC, actin will polymerize till the free monomer focus is diminished to the CC. For muscle actin at 4°C, the CC is roughly 0.03 mg/ml within the presence of two mM Mg²⁺ and 50 mM KCl. Within the absence of those ions, the CC will increase considerably, demonstrating how ionic circumstances affect polymerization. Equally, non-muscle actin displays various CC values relying on the ionic setting and temperature, reflecting its dynamic nature.
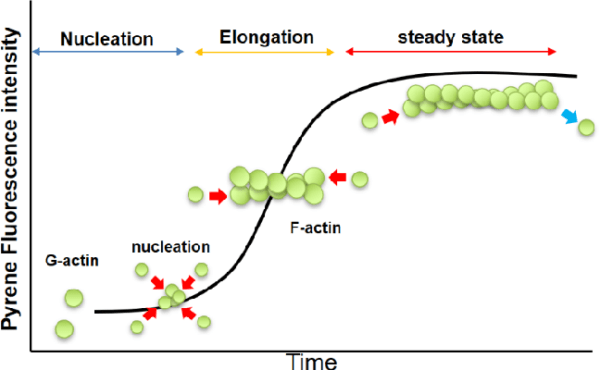
Measuring Actin Polymerization
A number of strategies are employed to measure actin polymerization, every with particular benefits and limitations:
- Fluorescence Enhancement of Pyrene Conjugates: This system includes utilizing pyrene-conjugated actin, which displays elevated fluorescence upon polymerization. The fluorescence enhancement is as much as twenty-fold, offering a delicate and versatile technique for monitoring actin polymerization in real-time. This technique is advantageous for finding out polymerization dynamics with minimal pattern disruption.
- DNase Inhibition Assays: This assay exploits the high-affinity interplay between G-actin and DNase I. G-actin inhibits DNase exercise, permitting for the differentiation between G-actin and F-actin. This assay is especially helpful for quantifying the quantities of monomeric and filamentous actin in cell extracts and finding out actin-binding proteins that generate G-actin from actin filaments.
- Viscosity Measurements: Viscosity measurements could be carried out utilizing excessive or low shear strategies, relying on the filament size. Excessive shear strategies are appropriate for detecting small variations between brief and medium-length filaments, whereas low shear strategies are used for longer filaments. Though these strategies can disturb the filaments and will not be extremely correct, they’re helpful for evaluating filament cross-linking between samples.
- Spin-Down Assays: Spin-down assays depend on differential sedimentation to separate F-actin from G-actin. This technique supplies quantitative information on actin polymerization at regular state. It may be utilized in mixture with different strategies to quantify actin polymer over time. Nevertheless, this technique is harmful and should overestimate the quantity of actin monomer on account of incomplete sedimentation of small actin oligomers.
Modified Actins and Their Makes use of
Modified actins, comparable to fluorescently labeled or biotinylated actins, are invaluable instruments in analysis:
- Fluorescent Actin: Fluorescently labeled actins are used to review actin dynamics each in vivo and in vitro. As an example, fluorescent actin could be microinjected into cells to look at actin treadmilling or myosin mobility. This method is helpful for understanding the habits of actin filaments in numerous mobile contexts.
- Pyrene Actin: Pyrene-conjugated actin is employed to watch actin polymerization. The elevated fluorescence of pyrene actin upon polymerization supplies insights into the dynamics of actin filament formation.
- Biotin Actin: Biotinylated actin has a number of functions, together with probing actin dynamics and selectively purifying actin utilizing streptavidin-coated beads. It will also be used as a probe for actin dynamics in cells when mixed with streptavidin or avidin-coated gold particles.
Actin Binding Proteins (ABPs)
Actin interacts with a various array of proteins, often called actin-binding proteins (ABPs), which considerably affect its operate. Over 150 ABPs have been recognized, constituting roughly 25% of mobile proteins. These proteins modulate actin dynamics and play essential roles in numerous mobile processes:
- Muscle Contraction: Actin filaments work together with myosin to facilitate muscle contraction. This interplay is important for muscle operate and motion.
- Cell Motility: Actin-driven actions are vital for cell migration, permitting cells to maneuver and alter form in response to varied alerts.
- Cytokinesis: Throughout cell division, actin filaments kind the contractile ring that separates daughter cells, guaranteeing correct cell division.
- Cytoplasmic Streaming: Actin networks help the movement of cytoplasm inside cells, contributing to varied intracellular processes.
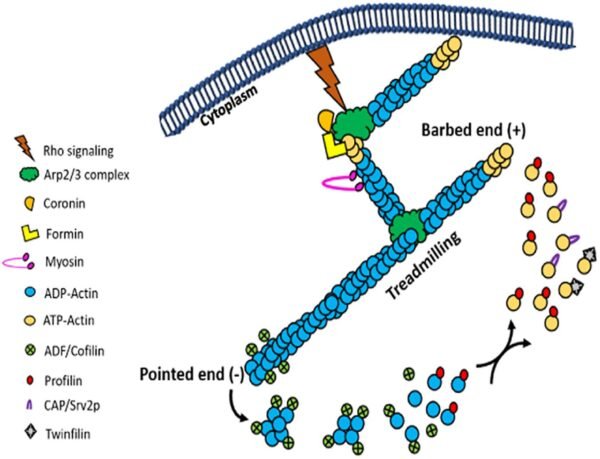
ABPs could be remoted and studied utilizing a spread of biochemical, genetic, and immunological methods. Strategies comparable to affinity chromatography and assays based mostly on pyrene actin fluorescence are instrumental in figuring out and characterizing these proteins.
Medical Significance of Actin Analysis
Actin analysis has profound medical implications, notably in understanding illnesses related to cytoskeletal dysfunction. Some key areas of curiosity embody:
- Erythrocyte Membrane Proteins: Mutations in proteins like spectrin, ankyrin, and band 3, that are concerned within the erythrocyte membrane skeleton, result in circumstances comparable to hereditary spherocytosis and elliptocytosis. These mutations have an effect on the form and stability of pink blood cells, leading to elevated fragility and lysis.
- Muscular Dystrophies: Dystrophin, an actin-associated protein, is important for linking the actin cytoskeleton to the muscle cell membrane. Mutations in dystrophin end in Duchenne and Becker muscular dystrophies, characterised by progressive muscle weak point and degeneration. Understanding dystrophin’s function in muscle cells is essential for growing therapeutic methods for these circumstances.
- Neurodegenerative Ailments: Proteins related to the actin cytoskeleton are additionally concerned in neurodegenerative illnesses. For instance, proteins that hyperlink the actin cytoskeleton to synaptic buildings are vital for sustaining neuronal operate. Dysregulation of those proteins can contribute to illnesses comparable to Alzheimer’s illness and different neurodegenerative problems.
Molecular Infrastructure and Dynamics
Actin varieties a posh ultrastructure that helps mobile form and movement. The cytoskeleton, composed of actin filaments, supplies a scaffold for mobile group and facilitates numerous mobile actions. Actin filaments are concerned within the formation of pseudopods utilized by amoebas for crawling and the microvilli in intestinal cells, which prolong into the digestive tract for nutrient absorption.
Actin’s dynamic nature is a trademark of its operate. Actin filaments are regularly assembled and disassembled because the wants of the cell change. This dynamic habits is regulated by ATP binding and hydrolysis. Free actin monomers bind ATP and affiliate with rising filaments. As ATP is hydrolyzed to ADP, the actin filament undergoes refined structural adjustments, resulting in a decreased stability and eventual disassembly. This dynamic course of, often called treadmilling, includes steady polymerization at one finish and depolymerization on the different, permitting the filament to “move” by the cell with out altering its general size.
Managed Development of Actin Filaments
In mobile contexts, actin filament development is tightly regulated to stop uncontrolled polymerization. Proteins comparable to gelsolin and profilin play essential roles in controlling actin dynamics. Gelsolin severs actin filaments, producing new ends for additional polymerization, whereas profilin binds to actin monomers, selling their incorporation into filaments. This regulation ensures that actin filament formation is conscious of mobile alerts and wishes, maintaining mobile integrity and function.
Future Instructions in Actin Analysis
Developments in actin analysis proceed to uncover new dimensions of its operate and regulation. Future analysis instructions embody:
- Structural Research: Excessive-resolution structural research of actin and actin-associated proteins will present deeper insights into their interactions and capabilities. Methods comparable to cryo-electron tomography and X-ray crystallography might be instrumental in elucidating these buildings.
- In Vivo Research: Using superior imaging methods, comparable to live-cell fluorescence microscopy and super-resolution microscopy, will improve our understanding of actin dynamics in dwelling cells and tissues.
- Drug Improvement: Focusing on actin dynamics and interactions with particular inhibitors or modulators holds promise for growing therapeutic methods for illnesses associated to cytoskeletal dysfunction.
- Methods Biology: Integrating information from numerous omics approaches will assist in understanding the complicated networks of actin and its interacting companions, offering a holistic view of its function in mobile processes.
In abstract, actin is a flexible and important protein concerned in numerous mobile capabilities. Its dynamic nature and interactions with quite a few proteins make it a central participant in cell biology. Ongoing analysis into its construction, operate, and regulation continues to supply invaluable insights into each fundamental mobile mechanisms and the pathogenesis of actin-related illnesses.

